Pharma Lions
The Pharma Lions celebrate creative communications for pharmaceutical clients and services with work that brings science and innovation to life.
Introduction

The history of the Pharma Lions
The Pharma Lions were launched in 2014, alongside the Health & Wellness Lions, to recognise creativity in a heavily regulated industry. It celebrates products and services prescribed by healthcare professions.
Since being introduced, the Award has evolved and redefined how we reflect this sector through constant open dialogue and development with the industry.
What kind of work can be entered?
All work entered into the Pharma Lions must be aimed at specific practitioners and patient groups. It must address the management of a disease or medical condition diagnosed and treated by a healthcare professional.
The work must be created specifically to shape understanding of medical conditions, drive their treatment and/or advocate for the development or provision of those treatments. Work that is for an OTC or general wellbeing product or service should be entered into the Health & Wellness Lions.
What’s the Jury looking for?
Bold, innovative ideas that navigate the unique challenges of pharmaceutical regulations while standing out creatively within the sector's constraints. This may include work that overcomes barriers such as legal compliance and complex medical language, prioritizing empathy and understanding of patient journeys to tell compelling stories about how treatments or innovations improve lives. Innovative approaches to visualizing data or medical conditions, as well as pioneering work that pushes beyond traditional norms to elevate the standards of communication in the pharma industry is also eligible.

HEART SURGEON'S COOKBOOK | GETINGE | FORSMAN & BODENFORS, GOTHENBURG | BRONZE, PHARMA LIONS | 2024
The Categories

To enter into Pharma Lions, you must first select the sector/category (direct to consumer, regulated, non-regulated and veterinary) for your piece of work. Then you select the relevant medium.
The categories in Pharma are representative of the sector and the mediums are reflective of the types, and the forms of the work being entered. The mediums very closely follow the specialisms of the Lions, for example, Brand Experience & Activation or Creative Data.
A. Direct to Consumer
B. Regulated
Work created for a specific regulated branded product, service, therapy or company to drive choice, progress healthcare advancement and raise awareness and understanding of a disease or medical condition.
Only work for products or services that must adhere to strict regulatory compliance and compulsory fair balance copy can be entered in section B. Regulated.
All entrants within this section must provide regulatory requirements for your region in relation to your submission.
C. Non-Regulated
Work created for a non-regulated branded product, service, therapy or company. Only work for products or services that are utilised by specific practitioners and patient groups in relation to the management of a disease or medical condition diagnosed and treated by a healthcare professional can be entered into C. Non-Regulated.
If you wish to enter an OTC or general wellbeing product or service, please see the Health & Wellness Lions.
D.Veterinary
Mediums
The mediums are a type of subcategory found in Health & Wellness Lions and Pharma Lions only and are chosen by the entrant in addition to the category for each submitted piece of work.
Eligibility

Work will need to have been executed during the eligibility period of 8 February 2024 – 10 April 2025.
Is there a limit to how many times I can enter?
There’s no overall limit to how many times the same piece of work can be entered into Pharma as long as the mediums chosen are relevant.
Exceptions:
- The same piece of work may be entered into the Pharma Lions or the Health & Wellness Lions.
- Only products and services prescribed by healthcare professionals should be entered in the Pharma Lions.
- The same piece of work can only be entered in either section 'A. Direct to Consumer’, ‘B. Regulated', 'C. Non-regulated' or 'D. Veterinary'.

MIS[S]DIAGNOSED | ORGANON | MULLENLOWE MENA, DUBAI | BRONZE, PHARMA LIONS | 2024
Previous Winners

Magnetic Stories
Cannes Lions 2024 Grand Prix Siemens Healthineers | AREA 23, an IPG Health Network Company, New York
Siemens Healthineers developed a series of audiobooks that transform the unsettling sounds of paediatric MRI scans into engaging narratives. By syncing the noises of common scans with creative storytelling, the initiative reduces anxiety for young patients, making MRIs a more enjoyable experience. Launched at CUF hospitals in Portugal, the project aims to expand across Europe, enhancing Siemens’ reputation as an innovative leader in healthcare.

MAGNETIC STORIES | SIEMENS HEALTHINEERS | AREA 23, AN IPG HEALTH NETWORK COMPANY, NEW YORK | GRAND PRIX, PHARMA LIONS | 2024
Why the work won
The Pharma Jury awarded this work the Grand Prix for improving patient experience and transforming a frightening medical procedure into an engaging and immersive experience. Jury President Collette Douaihy called it “storytelling at its best”.
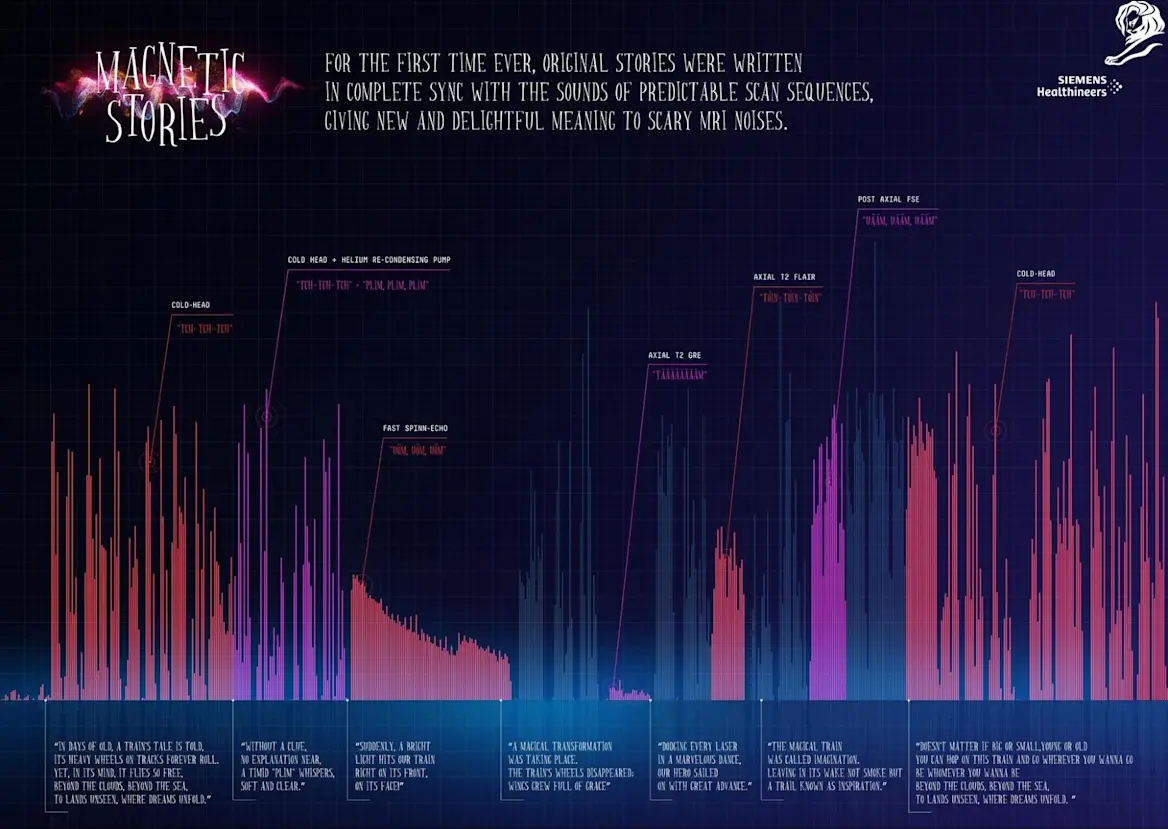
MAGNETIC STORIES | SIEMENS HEALTHINEERS | AREA 23, AN IPG HEALTH NETWORK COMPANY, NEW YORK | GRAND PRIX, PHARMA LIONS | 2024
Inside the Jury Room: Pharma Lions
To hear more from the Jury and how they decided on the winning work, check out the video below.
Entries & Judging

What are the judging criteria?
Individual judging criteria is dependent on the medium selected
What do I need to provide with my entry?
To enter, you’ll need to complete an online entry form, which can be found on the Cannes Lions entry site. In the ‘Written Explanation’ section, you’ll be asked to provide the Jury with everything they need to know about your work.
You’ll also be able to submit optional supporting materials to provide additional context with your entry.
Subtitles are highly recommended on all case films, films, demo films and original content.
Detailed information outlining the entry process can be found on A Guide to the Cannes Lions Awards.
What if I don’t have all the details right away?
Don’t worry – you can fill in the form in any order and save your progress at any time while it’s still in the pending stage. However, please only check out when your entry is 100% complete as no changes can be made to your entry once you’ve clicked “Submit” and “Checkout”.
The Written Submission

Here’s a preview of the questions you’ll be asked regarding your work.
To start your entry to the Pharma Lions, go to our Awards page.
The Jury will refer to the written explanation you provide throughout the judging process. Our advice is to provide as much relevant detail as possible.
When answering the following questions keep the main element you’re trying to celebrate in mind (e.g. the storytelling, the strategy, the craft, the use of data).
The bullet points under each question are prompts designed to guide your thinking, based on the factors the jury will be considering when reviewing your work. You might not need to use them all, and that’s totally fine. They’re there to help you consider what’s most important in your work.
Questions

NOT A LONELY JOURNEY | BIOGEN | VML, MILAN | BRONZE, PHARMA LIONS | 2024
Contact us

Everything you need to know to enter
For more detailed information on how to use the entry site, craft a compelling submission or submit your work we have a whole range of resources.
From Entry Guides that give you a step-by-step overview of the entire process to the Entry Kits that provide all the detailed rules and requirements for each and every category.

We are here to help
As Awards Experts, we know the Lions inside out. We can help with:
- Sharing the updates to the Awards for 2025
- Offering best-practice advice on how to craft a compelling entry
- Discussing your work and relevant categories
- Scheduling regular catch-ups to answer any questions that arise throughout the entry process.
Whether you have a specific query or would like expert advice, get in touch today.
Email: awards@canneslions.com Helpline: +44 0 20 7046 1155